The heart always intrigued people. One always wondered how such a small organ could generate so much power to contract and relax rhythmically for circulating blood and nutrients throughout the body. The heart was acting like a pump and people were mystified about the nature of spontaneity. Mythological stories were there. Hippocrates, (460-375 BC), observed that "those who suffer from frequent and strong faints without any manifest cause die suddenly". Aristotle, (384-322 BC), visualized that the heart linked the soul with life and was the source of all movement. The alternate contractile action of the heart moved the flow of blood in the body. The ancient Indian and Chinese practices in medicine emphasized deductive diagnosis by palpating and recognizing the subtle nuances in the pulse. Roman pagan medical practitioners unknowingly used to treat pain associated with acute gout with electricity emanating from electric rays or other such aquatic creatures.

Later, pulse diagrams and the theory, as described by Michael Bernhard Valentini in 1713, became popular for a short period. The relation of low pulse rate and syncope was hypothesized by Geronimo Mercuriale(1530-1606), in 1580. In the early 15th century there was a popular story saying that William Harvey is said to have restarted the arrest of a pigeon's heart by stroking the breast of the seemingly dead bird by a simple flick. Soon thereafter in 1628, he described the famous scheme of circulation for which he is famous. 1640 onward speculative publications that a bio-electric force was responsible for the automaticity in the cardiovascular system were there. In 1664, the Danish anatomist and naturalist Niels Stensen proved that the heart is a muscle. Static electricity was known to people from antiquity and was stored in a Leyden jar. Unrelated Stories about the effects of electricity on animals, mainly hens, circulated from time to time around this period. The first reference to external electrical stimulation of the heart is in the Registers of the Royal Human Society of London in 1774. The physician was Squires and the patient was a young girl. She became unconscious when she fell out of a window, she was taken to London's Middlesex Hospital and repeatedly shocked by a surgeon named Mr Squires. After he tried applying wires to various places on her body, he tried her chest, which caused her to begin breathing again and eventually wake up. There is a publication by the Danish physicist Nickolev Abildgaard (1775) claiming the death of a hen when an electric current was applied to its head. When electrodes were applied across the chest the bird was reanimated. Nothing happened after the application of these on other parts of the body.
Luigi Galvani's, (1737-1798), demonstration of bio-electricity in frog-leg nerve-muscle preparations followed by Alessandro Volta's, (1745-1827), discovery of a wet battery enabled medical researchers to venture into similar bio-electric properties of the cardiovascular system.

Alexander von Humboldt was a naturalist and he is said to have observed the flapping of wings of a dead bird in a futile attempt at resurrection when a jolt of electricity was given with one electrode, of zinc, within the beaks and the other, a silver shaft, in the cloaca. The rumour is also there that he tried the same on himself with an unpleasant experience. The demonstration that Galvanic current generated from a Voltaic pile affected the human heart was further studied. The gory experiments of Bichat & Nysten, 1800, on guillotined corpses, are best forgotten. Aldini in 1804 first demonstrated that syncope of cardiac origin can treated by "Galvanic energy". However, these were animal experiments only. It is said that Von Kollicker extensively studied the "action potential" in frog-heart in 1855 and hypothesized that electricity was produced with each contraction. Relating the different wave-forms and the regions of the heart was recognized later and the different

phases were influenced by the influx and efflux of ionic concentrations of Na+, k+, and Ca+ came out much later as the result of intensive research.
In 1872 Guillaume Duchenne de Boulogne, who advanced neurology and revived the science of electrophysiology, was said to have successfully resuscitated a drowned child with galvanic current by attaching one electrode to the leg and rhythmically tapping the intact pericardium with the other.

A unique opportunity came across Hugo Von Ziemssen in 1882. A female Prussian patient, Catherina Serrafin, had a large tumour excised along with the overlying ribs. She survived and the underlying heart on the left side, at this position, was covered with skin only. Von Ziemssen applied galvanic current to that area and recorded photographically the alterations of heart rate and the associated changes in rhythm. Though the experiment could have a fatal outcome, this was considered as the forerunner of cardiac electrophysiology and electrocardiography.

Walter Gaskell, in 1886, demonstrated specialized muscle fibres joining the atria and ventricles that caused "block" when cut and found that the sinus venosus was the area of first excitation of the heart. Serial section of embryonic tissue and its examination under a microscope led to the discovery of conducting tissue, the Purkinje fibres. These conglomerates of specialized myocytes were named after the Czech physiologist Jan Evangelista Purkyně, who discovered them in 1839 and are responsible for the conduction and propagation of the electrical stimulus causing automaticity. These distinctly identifiable tissues can be found in the sub-endocardial layers. The 'Bundle of His', a thickened path of Purkinje tissue from the AV node across the inter-atrial septum and into the intraventricular septum up to the branching, was discovered in 1893 by Wilhelm His jr. It was followed by the finding of the extra concentration of Purkinje tissue in the Koch's triangle of inter-atrial septum, the atrioventricular (AV) node. The AV node and the Bundle of His together with the right and left branches of the bundle in the septal tissue ultimately ramify in the ventricular muscle. The atrioventricular concentration of Purkinje tissue and its continuation across into the septum was found two years early by the Japanese Sunoa Tawara and a German Ludwig Aschoff. The sinuatrial node (SA node) is a concentration of Purkinje tissue at the posterolateral aspect of the right atrium and was found last and considered to start the automaticity in the cardiovascular system. The SA node was discovered by a medical student Martin Flack and with Arthur Kieth, his teacher, they published the same in 1907. To date, no specific pathway for intra-atrial conduction has been discovered and a generalized muscular propagation is suggested. The automatic electrical stimulus originates in the SA node and then rapidly propagates to the AV node by the atrial muscle. After a short delay in the AV node, the conductive stimulus propagates successively via the bundle and its branches inducing the heart to rhythmically contract. The event is complex and involves ionic gates and gap junctions. Complete recovery with reversal of ionic status occurs during the relaxation phase. The right bundle branch contains one fascicle. The left bundle branch is subdivided into two fascicles: the left anterior fascicle, and the left posterior fascicle. Other sources divide the left bundle branch into three fascicles: the left anterior, the left posterior, and the left septal fascicle. The thicker left posterior fascicle bifurcates, with one fascicle being in the septal aspect. An event-free life is possible with a block in the right bundle branch, however, a left anterior or a left posterior hemi-block is serious and implies atherosclerosis with coronary artery disease. Further ramifications of the conductive tissue occur in the sub-endocardial level in the heart muscle. Electrocardiography or ECG is the most common cardiac investigation done. The evolution of ECG is interesting. In 1885, Kollicker published his work on the 'action currents' of the heart and since then the intimate relationship of electricity to the heart has been appreciated. This was further strengthened successively by investigation by others. Variability of the heart rate and rhythm at will was first photographed by Ziemssen. Even heart blockage or a disruption in the conductive pathway and the various forms of atrioventricular blocks have been discovered. It was really in the latter part of the 19th century an Englishman named John MacWilliam collated the publications available and formulated the basics of rhythmic electrotherapy in feeble or arrested hearts. Augustus Desire Waller, a physiologist, made the breakthrough of discovering the ECG using the Lippmann capillary electrometer. He did it in a clinical-physiologic setting, acquired vast experience, and published reports. Alexander Muirhead was the first to record an ECG. History tells us that Muirhead only was capable of a static electronic recording of the heart in 1872. Ten years later, i.e. in 1882, Waller ingenuously moved a photographic plate onto which an oscillating light beam from the recording Lippmann electrometer hooked to the patient. This was the first real-time electrocardiography or ECG as it is commonly known. The ECG is a graph of voltage versus time of the electrical activity of the heart or as Waller called the "electromotive properties of the heart". Initially, this was a two-lead version and the electromeric cardiac waves had the four A, B, C & D distinct deflections within a cardiac cycle. Waller was an entertainer and had little regard for animal cruelty or any such concept. He lectured extensively and demonstrated the ECG of his pet bulldog applying one electrode on the chest of the experimental animal while dipping the other electrode-attached back leg in a container of saline. He was undaunted in his attitude but did not have an optimistic view about the prospect of ECG as a common cardiac investigation. Einthoven in the later and majority part of his investigations being interested in ECGs, used a string Galvanometer thus helping in somewhat reduction in the bulk of instruments, and also replaced the ABCD alphabets with P, Q, R, S, & T in the waves of ECG. He also introduced the concept of a triangle, the famous 'Einthoven triangle', and a graphical method of calculations and standardization in various conditions. 1895 is accepted as the time during which Einthoven pursued his interest in the electrical activities of the heart, but with the introduction of the string Galvanometer, he switched his focus and devised a more practical model for carrying out the pain-free non-invasive test. In 1927, General Motors came up with the first portable ECG. Wilson, in 1933, in addition to Einthoven and others' idea of the 3 standard leads, gave the idea of using properly placed 6 chest leads to make ECG diagnosis of myocardial wall motion more specific. Takemi in 1937 devised an even compact model of the portable machine. introduced the additional chest leads and Goldberger, in 1942, introduced the concept of augmented leads - thus 3 standard leads + 6 chest leads + 3 augmented leads = 12 lead ECG. Rune Elmqvist's contribution in the latter half of the 1940s requires mention and he developed the inkjet print mechanism that came with the instrument. The Second World War had its impact and post-war commercial production of compact portable machines was offered by many leading companies presently this investigation is one of the commonest performed to have an impression of the heart's health. While the relation between electricity and cardiac contractility was sorted, a parallel study was being done by several pioneers who tried to understand the mechanism of transient loss of consciousness and the heart. A search of the literature shows that a Slovenian named Gerbezius first pointed out, as early as 1717, that there was bradycardia with prolonged and persistent heart block. Later in 1761 Morgagni theorized a causal relationship between heart rhythm and sudden loss of consciousness and gave a clinical description of circulatory arrest. It was 1827, when the Irish surgeon Robert Adams related blackouts, called apoplexy at that time, with slow pulse rate. William Stokes in 1846 reaffirmed Adam's findings giving a detailed analysis. Henceforth, though the contributions of Gerbezius and Morgagni appear significant and Adam's was the first to describe the condition, for simplicity and convenience, the transient loss of consciousness due to a cardiac event is known as the Stokes-Adam's attack or the SA attack. The works of John MacWilliam in laying down the principles of electrotherapy of the heart of that time, Abildgaard's feat of reviving a so-called dead hen, and Ziemssen's unknowing stimulation of the heart of Catherina Serrafin by discharged current, led to the discovery of a defibrillator which was thought to be able to save death due to drowning and other such consequences as result of a misadventure. Automated and R-wave synchronized defibrillators are available at all modern places and even kiosks and guidelines dictate delivering shocks to patients who suffer sudden cardiac arrest from any cause. It has become part of an essential drill in cardiopulmonary resuscitation of modern times.

The sinuatrial node (SA node) by the highest rate among the conductive tissue also is the first to repolarise thus initiating cardiac automaticity. Sinus node dysfunction also called the sick sinus syndrome is characterized by phases of tachycardia and symptomatic bradycardia. A sinuatrial arrest leads to the non-production of 'p' waves with consequential drops in QRS complexes and the resultant transient loss of consciousness.
Paradoxically both first and second degrees of atrioventricular blocks were discovered without the help of ECG because it was not commonly available at that time. Wenkebach is said to have carefully examined diagrams of pulse to theorize the first-degree heart block in 1899. John Hay, from Liverpool, reported a case of a second-degree heart block critically examining both radial and jugular pulses. Mobitz, however, had the opportunity to use ECG and pulse diagrams, both arterial and jugular venous, to corroborate both Wenkebach and Hay's observations. A delay in the PR interval beyond 200 milliseconds but not resulting into a drop of the R wave is typical of first-degree AV block or the Wenkebach phenomenon. A further progressive delay in the PR interval with an ultimate absence of the R wave causes type I Mobitz block or the first variety of atrioventricular block. In the type II Mobitz block, the drop in R waves is more frequent and not related to the delay in AV nodal conduction. Third-degree heart block is characterized by complete atrioventricular conductive dissociation. The condition is also known as the complete atrioventricular block. On electrocardiography (ECG), a complete heart block is represented by QRS complexes being conducted at their rate and independent of the P waves. Ventricular tachycardia or junctional tachycardia also leads to atrioventricular dissociation but the reason for that is the sudden intrinsic higher ventricular rate than the native atrial rate. These are not considered as a form of complete heart block which can be of the following varieties:-
1. Regular P-P interval.
2. Regular R-R interval.
3. Lack of an apparent relationship between the P waves and QRS complexes.
4. More P waves are present than QRS complexes.
A complete heart block is usually characterized by severe bradycardia approximately around the idioventricular rate. Roughly 1/5th of the cases are found to have some derangement in the Purkinje tissue of the AV nodal tissue, 1/5th of cases involve the bundle, and 3/5th reveal a defect just beyond the branching of the bundle of His. Though correction and reversal of the initiating pathology, if possible, is the goal, a pacemaker is commonly implanted whenever the block is Phase IV for early effect.
The evolution of cardiac electrotherapy and pacemakers is interesting. A critical review shows that an Australian named Mark Lidwell in the year 1928 is said to have saved a child by applying intermittent electricity to the ventricle of the heart using an alternate current electricity generator. This is the only available report (Australian Congress) and there is no documentation of other cases. The next reference to an electric manipulation of the heart was found in 1932 onward and in the United States. Albert Hyman experimented with the arrested heart first with an injection of drugs and then with electricity. First, he injected drugs like adrenaline into the heart muscle and soon realized that an intra-cardiac injury current was needed for the initiation of a QRS complex which can cause an effective contraction of the ventricles. Unlike Lidwell, Hyman's electricity stimulator was fabricated by himself, with a hand crank option producing direct current. A clockwork mechanism was built into it and the heart stimulated at fixed 30, 60, and 120 per minute rates. He named this "intra-cardial (as documented by himself) therapy" a cardiac pacemaker and this name persist. He tried involving the United States Navy and was denied and became frustrated with time. Ultimately Siemens-Halske of Germany and the American subsidiary Adlanco produced the prototype and named it the 'Hymanomotor' with erratic results. There was no progress or interest in the Hymanomotor thereafter.
Bigelow and Callaghan, since 1949, observing hibernation in animals, experimented with hypothermia. They noticed a significant reduction in oxygen consumption and bradycardia signifying lowered metabolism. However, primarily they had their eyes on cardiac surgery. Soon they realized that early myocardial recovery was dependent on other factors as well.Early sinuatrial stimulation aimed at achieving a faster and stronger cardiac contraction was secondary and may depend upon an 'accidental poke' to the heart.
The late 1940s and early '50s saw the development of mains-powered and vacuum tube based direct current electric stimulators for the physiology labs for clinical experiments. The Grass stimulator (Grass Manufacturing Co) was one suitable for use to stimulate biological tissue. The advantage was that all three output parameters the stimulation rate, the voltage, and the pulse width could be varied. The pulse generated was monophasic, rectangular and had a duration of 2-20 ms. Based on this, John Hopp, an electric engineer, built a tabletop instrument that could convert alternate current into direct current that could be harnessed and delivered transvenous into the atrium of the heart for sinuatrial stimulation. The unpleasant effects of cardiac stimulation by the alternate current were avoided together with muscle twitching when the electrodes were applied to the atrial walls through an intercostal space. Furthermore, the procedure used a bipolar lead for atrial stimulation and the transvenous positioning did not require an invasive thoracotomy.
In 1951 Paul Zoll improved upon the biological tissue stimulators available then and modified an ECG machine to synchronize and monitor an electrical pulse of 2 ms duration only. The apparatus was bulky, 'mains' powered, and required a trolley for transportation. The output voltage ranged between 50 and 150 volts and an unpleasant alternating current was delivered with the help of two 3 cm² metallic electrodes strapped on the chest directly over the heart. Zoll's Electrodyne PM 65 having the advantage of synchronized monitoring, was an external electro-stimulator and by repeated bursts could salvage episodes of prolonged ventricular fibrillation. The trans-thoracic electrical stimulation was painful. Though these patients were sick, enduring these uncomfortable bursts prevented sudden death. Zoll reported his feats in 1952. Thereafter he was more interested in developing monitors for clinical use.
In 1956 at the St. George's Hospital in the United Kingdom, Aubrey Leatham asked his co-worker Geoffrey Davis to design a circuit that could initiate demand pacing utilizing
asystole interval to avoid probable R on T ventricular fibrillation occurring with the Zoll's instrument while trying to treat cardiac arrest. The first demand pacemaker was born and the commercial production (Firth-Cleveland, UK) had certain deviations and additions which included a depiction of the asystole interval, sensitivity settings of the tissue, and two output provisions. The instrument could deliver an electrical pulse trans-thoracic and at a potential difference of 150 volts. The beauty of the contraption was that for a short period, it could be operated by a battery.

The late '50s and early '60s of the 20th century were known as the golden years. Maximal groundbreaking therapy in cardiac electrotherapy occurred during this period.
The Maverick cardio-thoracic surgeon, Clarence Walton Lillehei had a problem. He was experiencing post-operative mortality due to the per-operative development of conduction defects in a percentage of cases that could not be revived. Earl Bakken used to repair hospital equipment and had his workshop in a garage she'd just outside the University hospital where Lillehei worked. At Lillehei's request, Bakken looked up old copies of the "Popular Science" magazine and came up with an idea. He constructed a transistorized battery-operated wearable electric pulse generator where the rate of pulse

generation could be altered at will and according to the sensitivity of the myocardium. Wearable meant a small battery-operated instrument that could be attached to the patient for ambulation. The story is that this new contraption was working perfectly in dogs in the animal lab of the hospital and when Bakken got there to check, he was surprised to find it hooked to one of Lillehei's young patients in the ward instead of the dog laboratory attached to a dog. Lillehei nonchalantly told him that he saw no reason for the delay and that the patient's life was more important. The real problem of introducing infection along the myocardial wires was minimized by Lillehei himself when he developed insulated myocardial wires and tunnelled a part of the wire before bringing it out of the skin. Lillehei's patients were affected by a power failure in the University Hospital affecting his patient. He prodded Bakken to produce something better and the result was the first transistorized medical equipment that was both battery-operated and 'wearable'. This was at a time when the belief was that electrotherapy was for temporarily tiding over cardiac arrest situations, either in real life or post-operative rhythm initiation and maintenance. The very next year, a Swedish Cardio-thoracic surgeon Ake Senning teamed up with Rune Ehlmqvist, a medical graduate turned electronic engineer, to implant the first miniaturized, hermetically canned, and zinc-mercury oxide battery-powered pulse generator in the body of one Arne Laarson who suffered 20-30 transient unconsciousness attacks and was almost perpetually incarcerated at the Karolinska institute. He was not capable of leading a meaningful life and credit should be given to his bold wife in coaxing Senning and Elmqvist to try out the new 'handmade' apparatus for a better quality of life on Laarson. The pulse generator was of the size of the Kiwi shoe polish can, widely advertised at that time. Two electrodes were needed - one myocardial and one indifferent. The first implantation did not last due to technical reasons and the second one was implanted within hours which was a success. However, till the advent and popularization of the transvenous access and new long-lasting biologically accepted power source, pacemakers in conducting disturbance therapy never picked up. Furman and Robinson are credited with the theory of transvenous access and its practical demonstration. Their presentation appeared in the Surgical Forum in 1958. Though in the title the term "intra-cardiac pacemaker..." was used, actually they meant venous access and approach to the endocardium with myocardial stimulation from inside. Wilson Greatbach, an electric engineer, serendipitously discovered the 'squeg' phenomenon by attaching a 1 Meg resistor instead of a 1 k one in Buffalo. This allowed a pause in the pulse duration necessary for recovery repolarisation and longer life as no current was drawn by the transistor during the pause. Rate change at will could also be done. Chardack was the head of Cardio-thoracic surgery at the University Hospital in Buffalo at that time. He supported Greatbach's circuit, tested the stability and predictability in his experimental animal laboratory, and then implanted a canned pulse generator with Greatbach's circuit in an aged patient with a history of syncopes. The procedure was in two stages - the first consisting of a thoracotomy for attachment of the myocardial leads and the second, implantation of the pulse generator with myocardial electrode attachment. Chardack, Gage, and Greatbach, the 'Bow tie team', reported 15 such cases in 1961.

The pacemaker as a long-term treatment for conduction defects was not popular initially. The Grass stimulator was used, initially, for 'giving the poke', as described by Bigelow, in the early days for electrically starting an arrested heart after cardioplegic infusion in open heart surgery. Later it was replaced by Zoll's instrument. Lillehei showed the importance of keeping myocardial wires attached in the post-operative period till the safety of maintenance of an inherent rhythm. The advent off Bakken's wearable transistorized device and Senning and Ehlmqvist's feat of implanting the self-made miniaturized pulse generator opened a new horizon. Trans-venous lead insertion with endocardial stimulation of the heart had already been established and dual chamber pacing of the atria followed by a short delay soon came. With its limitations, dual chamber pacing was considered 'physiologic' pacing (atrial synchronized ventricular pacing or VAT pacing mode, 1962, Nathan, Miami, US). There were improvements in biotechnology leading to the abandonment of epoxy canning and the use of NiCrCo alloy coiled and multi-strand wires as electrodes. Polyurethane was used as insulation and one saw more use of Titanium in hermetically sealing the electronic circuit. Rate-responsive sensors in the form of piezoelectric crystals within the sealed cans or microcircuits capable of recognition of minute changes in myopotentials or respiratory parameters associated with increased metabolic rate parameters were introduced into the pulse generator circuit. The sensing microcircuits of some models are in the lead tips as well and these can pick up the changes faster. Together with these came the long-life lithium-iodine battery. Literature reveals that the use of such long-life batteries in an implantable pacemaker was made by Greatbach and his team. Previous to this the longevity of pacemakers was only 3 years. The lithium-iodine battery allowed 7 to 10 years to lapse before a change in the pulse generator was contemplated and if the lead parameters measured during the process is acceptable, then only the pulse is changed. A few words need to be mentioned about nuclear pacemakers here. The batteries have a life expectancy of more than that of the individuals' longevity. However, the permission and the paperwork involved together with the perceived risk of radiation to users and patients made it unworthy of further production.

Dual chamber pacing was established in 1976 onward and due to the slight delay the physiological mimicry and synchrony of atrial and ventricular contraction made it immensely popular.

Electrodes of late, two long insulated spring-wound wires, are bipolar and single. Fixation can be active with a screw mechanism or passive with fish fin-like projections near the tip. Steroid elution of the contact zone minimises the inflammatory response and the consequential injury current. Capture management involves: -- 1. Secure contact with the myocardium at all times.
2. Minimal current drain.
3. Impedance within 300-1500 ohms.
The indications and guidelines were soon formulated. The class of indications in short were:- Class I -- where a pacemaker is truly needed for sustenance of life. Class II -- where pacemaker implantation may benefit the patient. Class III -- in situations where pacemaker implantation is not indicated and might harm the patient. The modes of pacemakers are based on a generic code known as NBG ( combined from the North American Society of Pacing and Electrophysiology and the British Pacing Group NASPE/BPG). This typically consists of 4 letters:- Letter 1. In the area being paced, A stands for atria, V stands for Ventricle, D stands for Dual, O stands for none Letter 2. In the area that is sensed -- A stands for atria, V stands for Ventricle, D stands for Dual, O stands for none Letter 3. The response of the pacemaker to sensing: O stands for none, I stands for inhibiting, T stands for triggering, D stands for dual Letter 4. Rate adaptiveness. O stands for none, and R stands for rate adaptiveness.
Letter 5. Multisite pacing - sites.
The modes are best explained as follows:--
Single chamber pacemaker -- here only the right ventricular apex is paced.
VOO modes - here V stands for the only chamber paced. The response to sensing is not there.
VVI mode - the Vs stand for both sensing and pacing of the chamber paced and I for the inhibition of pacing when a signal is sensed. This is the true demand pacing mode.
AOO and AAI modes - The only difference is A stands for atrium. Otherwise, all are as above. These modes are indicated only when the AV nodal, His bundle, and ventricular conduction tissue is intact.
2. Dual chamber pacemakers - both the atria and the ventricles are paced when necessary. Dual chamber pacemakers can further be subdivided into those having tracking and non-tracking capabilities.
The tracking modes are the DDD and VDD.
The non-tracking modes include the DDI and the DOO patterns.
The third capital letter denotes the sense and delivery of pacing, whether sensed and delivered, sensed and inhibited, or the sensing and delivery mode is switched off.
When a pacemaker is chronotropic competent, the letter R is included as a suffix indicating rate adaptation or responsiveness. The capital letter RDR is added as a suffix to indicate some cases of neuro-cardiogenic shock where only the rate of the adoptive function is not sufficient (Rate drop response). Usually, the letters 4 and 5 seldom require mention and are not seen when describing the mode of a pacemaker.
The usual indications of pacemaker implantation are as follows:-- 1. Sinus node dysfunction. 2. Acquired AV block. 3. Post myocardial infarction. 4. Congenital complete heart block. 5. Long QT syndrome. 6. Hypertrophic cardiomyopathy. 7. Heart failure.
The goal is simple: --
Prevention of syncopal attacks.
Maintenance of a regular rhythm within physiological limits.
Improvement in the performance of the heart.
The pacemakers can be temporary or permanent. Quick central venous catheterisation and endocardial placement of the disposable lead has become routine and many lives have been saved by temporary pacing. The cases where reversion to a regular rhythm does not occur and in elected cases, where a conduction defect has been already diagnosed, a permanent pacemaker is implanted. In temporary pacing, usually a disposable bipolar lead is introduced transvenous and the bare electrode metal ends are attached to an external pulse generator. In some post-operative open heart operations removable myocardial wire for connection to the external generator. Implantation of a permanent pacemaker may either be epicardial or endocardial. Permanent endocardial pacemaker implantation necessitates the creation of a subclavicular pocket in addition to the trans-venous lead positioning.
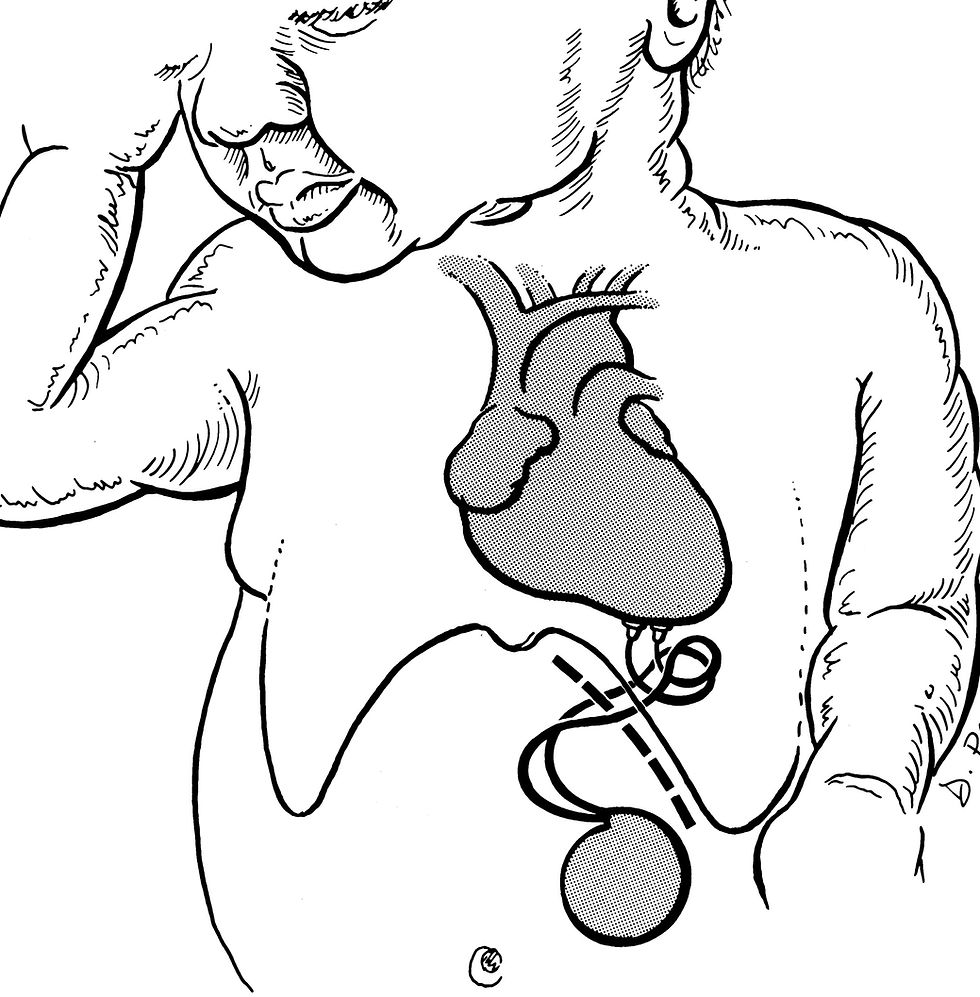
Epicardial pacing is routinely done in neonates and small children, and when there is a problem with the venous access. Endocardial pacing is always preferred as a morbid thoracotomy with the attendant problems are avoided. The leads are now normally bipolar meaning that an indifferent lead or the can itself acting as the indifferent lead is no longer required. It is to be remembered that pacemaker implantation is not indicated in an asymptomatic subject. Neuro-cardiogenic shock and the overactive carotid sinus in the neck require further investigation with the Head-up-tilt-table test (HUTT). A different type of pacemaker senses the R-R intervals and gives a pulse burst thereby preventing an arrest of more than 3 seconds and consequential low blood pressure and impaired cerebral blood flow. Such pacemakers are called rate drop pacemakers (RDR suffix) and these may be a bit different from the normal rate-responsive pacemakers such as the atrial-based single chamber (AAI-R) and the dual chamber (DDD-R). Neonatal pacemaker requirement is rare and may present as fetal bradycardia or detected by a sonologist during an intrauterine anomaly scan or a detailed intrauterine echo-cardiogram ordered by the obstetrician. Though a dedicated small model is available for babies, these are not usually available and in the majority of cases an adult pacemaker has to be implanted. In neonates and small children, epicardial pacing is the norm and a change to a more stable transvenous endocardial pacing is done the moment the central veins are large enough for accommodation of the catheter and allowing blood return at the same time. Normally owing to the higher metabolic activity, a rate below 50/min and chronotropic incompetence is risky. Still, asymptomatic neonates and children should not be paced. Finding a place for the creation of a pocket may be problematic. An extra loop of the endocardial portion of the lead in the right atrium is usually left behind to allow for growth when transvenous pacing is done in small children.

As mentioned, pacemaker implantation is never indicated in the asymptomatic patient and these may be :-
1. Syncope of undetermined etiology.
2. Sinus bradycardia without significant symptoms. 3. Sinuatrial block or sinus arrest without significant symptoms 4. Asymptomatic prolonged PR intervals with atrial fibrillation 5. Asymptomatic bradycardia during sleep 6. Asymptomatic 2nd-degree Mobitz I (Wenckebach ) AV block 7. Right bundle branch block with left axis deviation without syncope or other symptoms compatible with intermittent AV block 8. Reversible AV block such as those with electrolyte abnormalities, Lyme disease, sleep apnoea, etc.
Mandatory rest is always recommended and heavy weight lifting with getting near to high electro-magnetic fields have to be avoided always.
Implantation-related complications are the following: --
Pneumothorax.
Perforation of right ventricular apex or inferior wall with pericardial tamponade, diaphragmatic twitching, and or pericarditis and effusion in the long run.
Muscle twitching near and at the implantation site, upper arm of that side.
Hematoma at the implantation site.
Infection with a non-healing ulcer, chronic discharging sinus, or exposure to the pulse generator.
Major venous obstruction and related problems on the side of obstruction.
Lead dislodgement.
Lead fracture.
Twiddler syndrome (rarely seen and is likely to affect subjects with psychological issues, leads not actively fixed, and when myoclonic contractions of the pectoral group of muscles rotate the pulse generator in such a manner that the lead wounds up against the pulse generator).
A timeline of complications, as listed below, is normally accepted:--
Within 24 hours -100% technical or procedural Issues, like lead dislodgement/Screws and nuts.
Within 1-2 weeks – Again technical,-- Pocket issues, Infections.
Within 6 months – Benign pacemaker syndrome,-- Threshold settings, Scars
After the first year – Generally, issues are rare,--Lead issues, and associated disease progression.
In case of implantation problems and redo situations the cardio-thoracic surgeon is almost always called and may land with a complicated situation such as shown :



There is a condition called 'pacemaker syndrome' where the features suggest a possible intolerance to induced cardiac electrical activity. The symptoms include:--
Cannon A waves.
Chest pain.
Confusion, dizziness, and fatigue.
Palpitations.
Breathlessness.
Repetition of syncopal attacks.
It is nowadays believed that a problem in the conduction in the atrioventricular nodal tissue, both sub-optimal synchronous and dyssynchronous conduction, is the reason and is mainly seen in single chamber ventricular pacemakers.
Patients implanted with pacemakers are always aware that the heartbeat within is supplemented by a device that is within the body and in most cases connected by a specially constructed wire called the lead. A pacemaker checkup is recommended:
After 24 hours following implantation.
After six weeks following implantation.
Three months after the last visit.
Six months after the 3rd-month visit.
One year after the 6th-month visit.
On every succeeding year.
The pacemaker is checked as a routine for its:
Battery level.
Lead function.
Specific programming depends on the condition of the patient’s heart.
Performance -- at rest and during times of activity.
The implanting physician must inform the patient and the party about the frequency and nature of surveillance needed. The earlier recommendation was a 4-6 week initial check followed by checks every 3-12 months. However, this depends on the device implanted and the expected time the battery of the generator is going to last. With the stability of electronic circuitry and the long life of batteries, an implanted pacemaker is supposed to last 10-12 years in a single-chamber device and more than 7 years in dual-chamber devices. A more relaxed attitude and a yearly check earlier if required is always recommended now if the initial check indicates nothing wrong with the pulse generator and the lead. Pacemaker surveillance was in the subconscious thought of both the implant team and the patient since the day implantation was done to regulate the rhythm of the heart. This was done to prevent sudden death due to pacemaker failure and even elective replacements were done at a 3-year interval. Earlier a pacemaker recipient used to visit the implant surgeon and a trained agent of the company with a 12 lead ECG with and without a magnet for followup-evaluation. When a magnet is placed near the device, the pacing mode changes from the programmed mode to DOO, VOO, or AOO, and the pacing rate changes to a pre-determined company-decided rate. Placing a magnet near the device suspends tachyarrhythmia detection. When the magnet is removed, the device returns to its programme operation.
Essential information to be recorded
1. Pacing lead impedance.
2. Sensed P/R wave amplitude.
3. Pacing threshold.
4. Battery voltage and impedance.
A hysteresis with a minimal rate may be added and helps in pacemaker syndromes.
Magnets can be used to protect the pacemaker-dependent patient during diathermy, electrocautery and other sources of pulsed electromagnetic induction (EMI). In recent times of rampant use of communication devices and other digital equipment, electromagnetic and radio frequency waves are plenty and around us throughout. A magnet placed over a pacemaker opens a reed switch which makes the pulse generator discharge at a prefixed rate (AOO or VOO). In programming, ECGs were compared with and without a magnet and a recent 12-lead ECG. An effort was made to identify dysrhythmias like mismatched firing by the pulse generator or missed impulses. The method was crude and proved inadequate with the advent of the long-life lithium iodine batteries. The reed switches were replaced by Hall sensors to prevent erratic electromagnetic interaction and thus magnetic action is localized to a particular region of the pulse generator and difficult. Portable programmers were soon developed and this had a circular wand with a hole inside. The wand allowed wireless 'interrogation' with an implanted pacemaker when placed over the intact skin and communicated with the bulky programmer that had to be connected to the mains. The programmer is loaded with multiple algorithms and changes in the rate, assessment of the lead parameters, battery status, change in atrioventricular conduction delay, change of mode, switching on or off an attribute (like rate-tracking and anti-tachycardia measures, rate adaptivity, etc.) and can provide backup pacing and monitoring as well. Recent programmers are even small in size like that of a tablet with a slot for a wand in the back. Most important is that it is battery-operated, wireless, and lightweight.
The influence of chronotropic incompetence in the evolution of heart failure is
important.

The idea of treating heart failure situations in dilated cardiomyopathy or similar states with augmentation of the dual chamber pacing by stimulating the left ventricle in synchrony with the right ventricle as well to increase the force of overall contraction of the heart was unique. This form of bi-ventricular and atrial pacing is known as cardiac re-synchronisation therapy (CRT). Back-up ventricular pacing is a feature of both these conditions. Cardiac re-synchronisation therapy was first used in a clinical set-up in 1996. Classic indications included an ejection fraction of ≤35% and a QRS duration of ≥ 120 ms. 2001 is the year when CRT was approved for treatment in advanced heart failure. The procedure, now catheter-driven, is less invasive, better tolerated, and helps those patients in whom the ultimate answer is a heart transplant. Parallel work continued with the goal of having a narrow QRS complex with pacemakers. AV nodal pacing, His bundle pacing and left bundle branch (LBBB) pacing can do this.

The recent programmers can interrogate and assess the newer implantable devices as well.
I had the opportunity to assist in my first pacemaker in a patient who was quite senior by then and a practising family physician in our mofussil town. He had been already implanted 5 times previously and the last time was epicardial with an abdominal pocket. We were fortunate that no thoracic exploration for lead replacement was necessary as the lead parameters were within accepted limits. I have, thereafter, implanted countless pacing devices, ICDs, and re-synchronisation devices (CRTs and CRT-Ds) independently and have seen and managed a fair share of the complications. The procedure should never be taken lightly, decision is critical and meticulous asepsis is to be maintained. In redo or complication management situations pragmatism, common sense, and procedure limited to the minimum suffice in most conditions. Over the years there have been vast developments in pacemaker circuitry, changes in the pause times, atrioventricular communication, and the use of better bio-materials acceptable to human tissue. Cardiac re-synchronization therapy (CRT) with a pacemaker and an ICD. This device may be called a CRT-D when the left ventricular requires additional attention and such devices are recommended for people with heart failure who also have a risk of sudden cardiac death. It can detect dys-synchronous atrioventricular and ventricular beats. This dys-synchrony in beats can lead to dangerous heart rhythms, and the device will deliver a stronger shock of energy than a pacemaker can deliver to restore the steady rhythm to reset the heartbeat. In simple words, a shock is delivered to reset the whole rhythm system.
There is a change in the battery technology as well with these devices. It was realized that a greater quantity of charge had to be stored in a suitably made capacitor for a sudden discharge over a high potential difference in a very short period. initially, Vanadium pentoxide became the material of choice, however, nowadays Lithium Manganese Dioxide (Li/MnO2) having both better efficiency and longer life is preferred.
In advanced areas, an automated defibrillator is available everywhere, even in stores and kiosks, to save lives in sudden cardiac arrest situations. Before off-pump surgery became common, AC fibrillators were used for induction of ventricular fibrillation to avoid cross-clamping the aorta and completing an anastomosis fast. This, however, did not appear popular due to the biopsy appearance of extensive sub-endocardial infarction in these individuals.
The cost is high and higher is the cost with additional features. Physicians want MRI-compatible pacemakers now as MRI helps in better tissue diagnosis and differentiation. With the introduction of MRI conditional systems, the changes involved the use of non-ferromagnetic alloys for the circuitry and the can. The industry ensures that only MRI conditional pacemakers are available in the market and the patient with the party has to buy it at a higher price. Factors that affect the patient in MRI are: --
1. The static magnetic force.
2. The radio-frequency wave (RF), and
3. The gradient wave.
The conjecture is that these, separately or together, may cause flow generation and stimulation of the myocardium leading to a range of ECG changes, arrhythmias, and even induction of ventricular fibrillation. Implanted prostheses and metallic vascular clips may be dislodged, and implanted ferromagnetic materials can heat up to burn the surrounding tissue. The device may be made with"MRI safe"material", but that does not mean the equipment as a whole is "MRI compatible". So, the safe option is advising an MRI-compatible pacemaker whenever needed.
The Government hospitals have an agreement with a company whereby a single chamber rate responsive model is supplied that delivers a pulse on demand. These are not truly MRI compliant and an agent of the company will place a magnet over the pacemaker so that it will not affect the circuit with strong electromagnetic or radio-frequency waves. It was a fact I had to face in clinical practice during the active days that a number of my open heart patients came with an MRI already done and an observational study showed minimal effect in MRIs of 1.5 Tesla. In most centres, the recommendations of the internationally accepted guidelines are not followed and it is the patient who is going to suffer and be blamed. These types of equipment are made of 'MRI safe' bio-materials and we know that stainless steel, which is used for binding the sternum, is least affected by the RF and the magnetic waves of MRI.
A conclusion would be incomplete without this story --- Arne Larson, in whom the first pacemaker was implanted, outlived the pair of Ake Senning and Rune Elmqvist, the implanting surgeons and the developer. In his lifetime he received 5 pulse generators while 22 lead systems had to be changed. Evolution and development of pacemakers remain ongoing and in the recent past, there have been reports of successful implantation of lead-less pacemakers. The pacemaker was placed in the right ventricular apex in the single chamber pacing. Dual chamber pacing involved the introduction of two pacemakers, one pacing the atria as it was placed in the right atrial appendage. At the same time, the other was positioned in the right ventricular apex. Communication between the two occurred by a wireless mechanism and a physiological pacing with appropriate atrioventricular delay is maintained. The effort in the research and consequent development with the addition of new algorithms has always been at trying for a better quality of life in compromised hearts.
留言